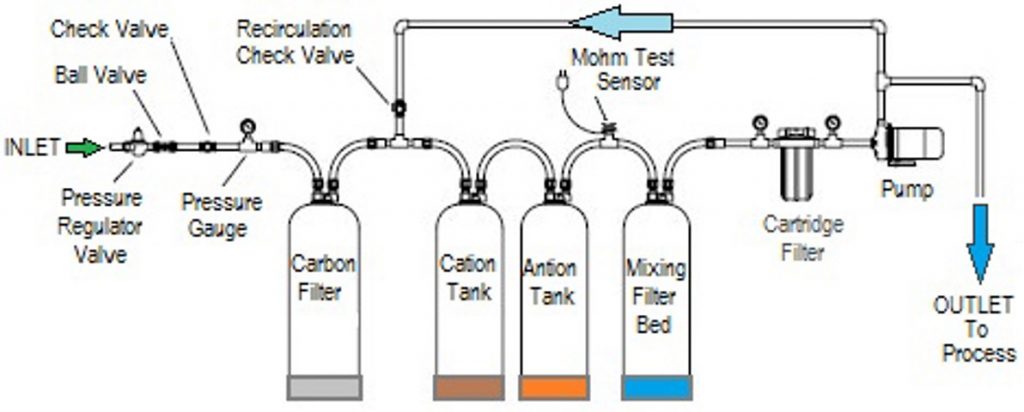
Deionization (DI) is the removal of all ionized minerals and salts (both organic and inorganic) from a solution through the process of ion exchange. Because most non-particulate water impurities are dissolved salts, deionization produces a high purity water that is generally similar to distilled water. Compared to distillation, deionization is faster, less energy-intensive, and more cost-effective. In addition, it is an on-demand process that supplies highly purified water as needed — an important feature because water at extreme levels of purity degrades quickly.
Deionization filters operate by exchanging positive hydrogen and negative hydroxyl molecules for the positive and negative contaminant molecules in the water. Positive chemicals like sodium, calcium, iron, and copper change places with the hydrogen molecules, and negative chemicals like iodine, chloride and sulfate change places with the hydroxyl molecules. This process is typically done by two ionized resin beds that are opposite in charges: cationic (negative) resin and anionic (positive) resin. Positively charged ions are removed from the solution by the cation resin in exchange for a chemically equivalent amount of hydrogen ions. Negatively charged ions are removed by the anion resin in exchange for a chemically equivalent amount of hydroxide ions. The hydrogen and hydroxide ions introduced in this process unite to form pure water molecules.
Deionized (Dl) water is an essential ingredient in hundreds of applications, including medical, laboratory processes, pharmaceuticals, cosmetics, electronics manufacturing, food processing, plating, countless industrial processes, and even the spot-free rinse water at the local car wash. Typically, it serves as an ultra-pure ingredient, a “perfect” cleaning solvent, or as the foundation of a process water recovery/reuse strategy.
